Production plants, machines and components
Customized production lines, machinery and plant components for your OTC-sweets, cannabis edibles, OTC medicines and supplements with active ingredients
As a specialist in moulding lines, we can cover the entire spectrum of deposited OTC-sweets and supplement confectionery with a wide variety of applications and capacities. Our plants are customer-specifically optimized to ensure the best possible product quality with maximum efficiency and profitability. The exact dosing of active additives and the fulfilment of GMP conformity for quality assurance is our main focus.
Characteristics:
-
Hygienic design
-
Simple construction principle
-
Free access for cleaning, disinfection and check
-
No hidden construction areas
-
Application of the 3D rule
-
High demands on the surface quality
-
Good emptying of residues
-
Short dwell times of mass, level-controlled mass supply,
-
First-in-first-out concept
-
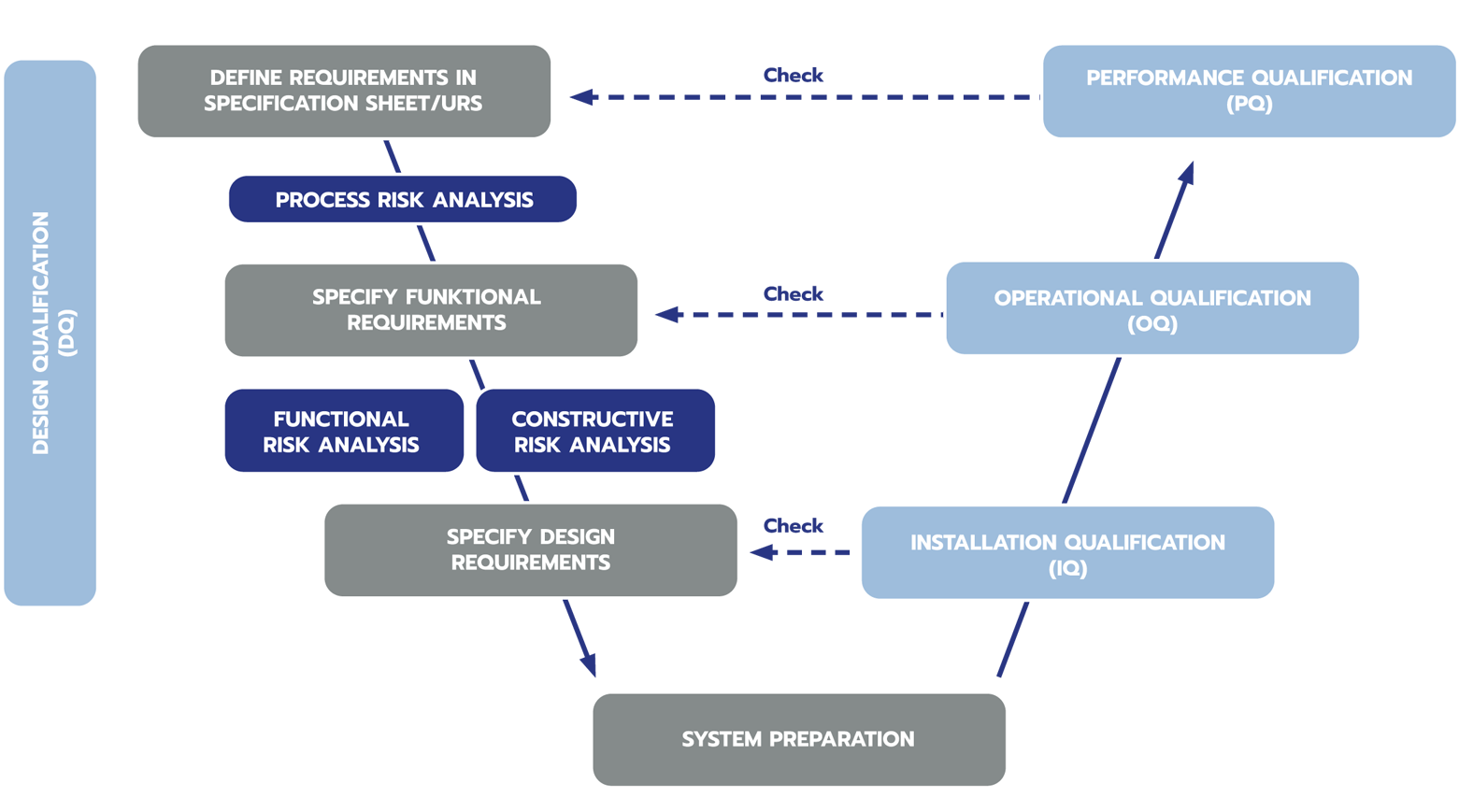
Risk-based approach across the entire development and qualification phase (DQ / IQ / OQ / PQ)
Plants for jelly products as well as for fondant, toffee and chocolate products
Our production plants are suitable for active OTC confectionery based on pectin, gelatine, starch, carrageenan and agar-agar. Confectionery masses such as fondant, toffee or chocolate are also suitable for the application of food supplements and other active substances. Depending on the requirements in the GMP environment, the masses are deposited according to high hygienic standards in solid and washable moulds consisting of silicone or polycarbonate or dosed in mould starch on Mogul plants. Moulding processes in polycarbonate moulds have gained popularity in recent years due to their effectiveness, productivity and hygiene.
More information about our plants.
Good manufacture practice – Safety for products with biologically active additives and OTC medicines
When adding substances that have an impact on the human organism, strict legal and hygienic requirements do apply.
In addition to high-precision cooking, dosing, cooling and moulding processes, these products require a suitable GMP concept (good manufacture practice) for the entire plant, i.e. precise and documentable dosing of the additives, effective and hygienic cleaning and the avoidance of cross-contamination.
Plant qualification in accordance with applicable GMP regulations
- DQ – Design Qualification
- IQ – Installation Qualification
- OQ – Operational Qualification
- PQ Performance Qualification
To avoid cross contamination e.g. fruit gums with biologically active additives, also called medical gums, or OTC medicines can only be deposited in washable silicone moulds or directly into packaging
The FFP process (Fast Forward Process), co-developed by WDS, therefore works with conventional or commercially available gelatine, which, thanks to its special composition, has fast setting properties, which is primarily responsible for the texture and haptic properties later on.